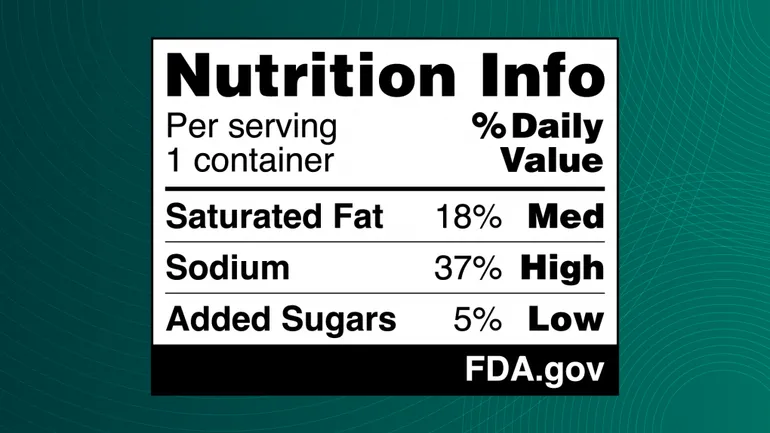
The U.S. Food and Drug Administration has recently proposed a front-of-package nutrition label requirement for most packaged foods. This label, also known as a nutrition info box, would provide consumers with visible information on a food’s saturated fat, sodium, and added sugars content, which are directly linked to chronic diseases when consumed excessively. The agency is currently accepting public comments on this proposal until May 16.
If finalized, the rule would classify products as having low, medium, or high levels of these categories, making it easier for consumers to make informed choices about their food purchases. Food manufacturers with annual sales exceeding $10 million would be required to implement the nutrition info box on their packaging within three years of the rule’s effective date, while smaller businesses would have four years to comply.
The FDA’s Nutrition Center of Excellence emphasized the urgency of addressing diet-related diseases and stated that the proposed rule has been a long-standing priority for the agency. This initiative aligns with government nutrition priorities aimed at combating chronic diseases, which are significant drivers of the nation’s annual healthcare costs.
Jim Jones, FDA’s deputy commissioner for human foods, expressed the agency’s goal of promoting wellness through food and potentially encouraging manufacturers to reformulate products to be healthier in response to the front-of-package nutrition labeling. The FDA’s research efforts and consumer focus groups have informed the design of the proposed label, with an experimental study indicating that a black and white “Nutrition Info” design with the percent “Daily Value” was most effective in helping consumers identify healthier food options.
In addition to the front-of-package nutrition labeling initiative, the FDA has recently updated its regulations on food products labeled as “healthy.” The agency is also working on developing a symbol that manufacturers can use to indicate a product’s “healthy” status on packaging.
While the Biden administration has yet to deliver updates to the Green Guides regarding environmental marketing claims, such as “sustainable,” “recyclable,” or “compostable,” the FDA’s ongoing efforts reflect a commitment to promoting informed consumer choices and addressing public health concerns related to nutrition and food labeling.